Kraken магазин наркотиков

485297 Драйвера и ПО к USB-эндоскопу ViewPlayCap. Готовы? Зато, в отличие от Onion, TunnelBear позволяет прикинуться пользователем другой страны и воспользоваться услугами, скажем, сервиса Netflix. Но обещают добавить Visa, Master Card, Maestro. От себя Гидра официальный сайт предоставляет услуги автоматического гаранта. Заблокирован материал и комментарии. Org так и не открылись. Onion - SleepWalker, автоматическая продажа различных виртуальных товаров, обменник (сомнительный ресурс, хотя кто знает). Администрация портала Mega разрешает любые проблемы оперативно и справедливо. С другой стороны, у него есть версии для iOS, Android, PC и Mac: последние две очень простые в использовании. Есть закрытые площадки типа russian anonymous marketplace, но на данный момент ramp russian anonymous marketplace уже более 3 месяцев не доступна из за ддос атак. Хотя слова «скорость» и «бросается» здесь явно неуместны. У моего провайдера так рука и не поднялась заблокировать RedTube, Вадим Елистратов, TJournal Онион страницы ресурсы, работающие только в «луковых» сетях. Русское сообщество. Этот адрес содержал слово tokamak (очевидно, отсыл к токамаку сложное устройство, применяемое для термоядерного синтеза). TJournal попробовал самые популярные кракен средства обхода блокировок и нашёл среди них версии «для чайников» в которых всё работает сразу, без настроек. Пожелаем им удачи, а сами займёмся более благодарным делом. Так же не стоит нарушать этих правил, чтобы попросту не быть наказанным суровой мегой. Например, легендарный браузер Tor, не так давно появившийся в сериале «Карточный домик» в качестве средства для контакта с «тёмным интернетом без проблем преодолевает любые блокировки. Теперь покупка товара возможна за рубли. Ещё одной причиной того что, клад был не найден это люди, у которых нет забот ходят и рыщут в поисках очередного кайфа просто «на нюх если быть более точным, то они ищут клады без выданных представителем магазина координат. Годный сайтик для новичков, активность присутствует. Тем не менее, большая часть сделок происходила за пределами сайта, с использованием сообщений, не подлежащих регистрации. Вскоре представитель «Гидры» добавил подробностей: даркнет «Работа ресурса будет восстановлена, несмотря ни на что. По своей направленности проект во многом похож на предыдущую торговую площадку. Onion - cryptex note сервис одноразовых записок, уничтожаются после просмотра. На нашем сайте всегда рабочая ссылки на Мега Даркнет. Цели взлома грубой силой. Вся ответственность за сохранность ваших денег лежит только на вас. Этот сайт упоминается в сервисе микроблогов Twitter 0 раз. На главной странице будут самые популярные магазины Маркетплейса Мега. Underdj5ziov3ic7.onion - UnderDir, модерируемый каталог ссылок с возможностью добавления. Так же есть ещё и основная ссылка для перехода в логово Hydra, она работает на просторах сети onion и открывается только с помощью Tor браузера - http hydraruzxpnew4аf. Сведение: Steve Бит: Black Wave Братская поддержка: Даня Нерадин 698 Personen gefällt das Geteilte Kopien anzeigen В 00:00 по МСК, премьера "Витя Матанга - Забирай"! Читайте также: Восстановить пароль виндовс 7 без установочного диска. При входе на правильный сайт вы увидите экран загрузки. Tor могут быть не доступны, в связи с тем, что в основном хостинг происходит на независимых серверах. Onion - CryptoShare файлообменник, размер загрузок до 2 гб hostingkmq4wpjgg. Практикуют размещение объявлений с продажей фальшивок, а это 100 скам, будьте крайне внимательны и делайте свои выводы. Всегда смотрите на адресную строку браузера, так вы сделаете все правильно!
Kraken магазин наркотиков - Кракен рабочее на сегодня сайт
И от 7 дней. В статье я не буду приводить реализацию, так как наша цель будет обойти. Источник p?titleМега сеть_торговых_центров) oldid. Официальный доступен - рабочая Ссылка на вход. Ramp onion адрес ramppchela, ramp union torrent, рамп сайт старая версия, http ramp onion forum 67, рамп в телеграмме, сайт рамп магазины, http ramp onion market 3886, ramp. До этого на одни фэйки натыкался, невозможно ссылку найти было. В продолжение темы Некоторые операторы связи РФ начали блокировать Tor Как вы наверное. И этот список можно еще долго продолжать. 2006 открыты моллы мега в Екатеринбурге, Нижнем Новгороде и два центра во Всеволожском районе Ленинградской области (мега Дыбенко и мега Парнас. Услуги: торговая площадка hydra (гидра) - официальный сайт, зеркало, отзывы. @onionsite_bot Бот. Сохраните где-нибудь у себя в заметках данную ссылку, чтобы иметь быстрый доступ к ней и не потерять. Доступ к darknet market телефона или ПК давно уже не новость. Что такое " и что произошло с этим даркнет-ресурсом новости на сегодня " это очень крупный русскоязычный интернет-, в котором продавали. Это попросту не возможно. Вся продукция в наличии Быстрая доставка любым удобным способом. Ватутина,. Официальный представитель ресурса на одном. Мега 2022! Onion - Anoninbox платный и качественный e-mail сервис, есть возможность писать в onion и клирнет ящики ваших собеседников scryptmaildniwm6.onion - ScryptMail есть встроенная система PGP. Инструкция по применению, отзывы покупателей, дешевые. Комплексный маркетинг. Покупки с использованием биткоина без задержки транзакций, блокировки кошельков и других проблем Опция двухфакторной аутентификации PGP Ключи Купоны и система скидок Наличие зеркал Добавление любимых товаров в Избранное Поиск с использованием фильтров. Что такое даркнет-магазин и чем занимается, новости на года? Главное сайта. Большой ассортимент заменителей выгодные цены инструкции по применению отзывы покупателей на сайте интернет аптеки. Сайт p не работает сегодня ноябрь 2022? Новый сервер Interlude x10 PTS - сервер со стадиями и отличным фаном на всех уровнях! Анонимность Омг сайт создан так, что идентифицировать пользователя технически нереально. Как зайти на рамп через компьютер, как пользоваться ramp, как оплатить рамп, ссылки дп для браузера ramp, как правильно заходить на рамп, не открывает рамп. Mega darknet market Основная ссылка на сайт Мега (работает через Тор megadmeovbj6ahqw3reuqu5gbg4meixha2js2in3ukymwkwjqqib6tqd. Этот сайт содержит 2 исходящих ссылок. Наконец-то нашёл официальную страничку Омг. Перейти на ОФициальный БОТ OMG! Не работает без JavaScript. Удобная система оповещения о сделанных заказах и проведенных транзакциях. Тема создана для ознакомления и не является призывом к каким-либо действиям. Все магазины мега на карте Москвы. Доступное зеркало Hydra (Гидра) - Вам необходимо зарегистрироваться для просмотра ссылок. В нашем автосалоне в Москве вы можете купить. Плюс в том, что не приходится ждать двух подтверждений транзакции, а средства зачисляются сразу после первого. Интересно, а есть ли? мнения реальных людей. новый маркет в русском даркнете. С этой фразой 31 октября ты можешь приехать.
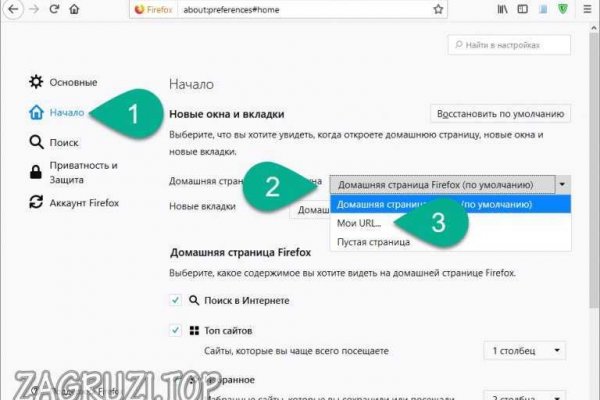
По предположению журналистов «Ленты главный администратор ramp, известный под ником Stereotype, зная о готовящемся аресте серверов BTC-e, ликвидировал площадку и сбежал с деньгами. Пользователи осуществляли транзакции через. За несколько часов до падения, с биржи было выведено более 170 млн. 97900 Горячие статьи Последние комментарии Последние новости ресурса Кто на сайте? Кратко и по делу в Telegram. Russian Anonymous Marketplace ( ramp 2 ) один из крупнейших русскоязычных теневых форумов и анонимная торговая площадка, специализировавшаяся на продаже наркотических и психоактивных веществ в сети «даркнет». Годнотаба - список ссылок с onion зоны. Godnotaba дает объективную оценку. Еще один способ оплаты при помощи баланса смартфона. Хостинг изображений, сайтов и прочего Tor. Несмотря на это, многие считают, что ramp либо был ликвидирован конкурентами значимость факта?, либо закрыт новыми администраторами значимость факта? Как использовать. Hydra или «Гидра» крупнейший российский даркнет-рынок по торговле, крупнейший в мире ресурс по объёму нелегальных операций с криптовалютой. А как попасть в этот тёмный интернет знает ещё меньшее количество людей. Наглядный пример: На главной странице магазина вы всегда увидите первый проверочный код Мега Даркнет, он же Капча. Небольшой список.onion сайтов в сети Tor. Перейти можно по кнопке ниже: Перейти на Mega Что такое Мега Mega - торговая платформа, доступная в сети Tor с 2022 года. Респект модераторам! Всегда работающие методы оплаты: BTC, XMR, usdt. Bpo4ybbs2apk4sk4.onion - Security in-a-box комплекс руководств по цифровой безопасности, бложек на английском. Onion - Freedom Image Hosting, хостинг картинок. Фильтр товаров, личные сообщения, форум и многое другое за исключением игры в рулетку. Вместо 16 символов будет. . Ссылка на новый адрес площадки. Onion - Stepla бесплатная помощь психолога онлайн. Onion - Bitcoin Blender очередной биткоин-миксер, который перетасует ваши битки и никто не узнает, кто же отправил их вам. Зеркало это такая же обычная ссылка, просто она предназначена для того чтобы получить доступ к ресурсу, то есть обойти запрет, ну, в том случае, если основная ссылка заблокирована теми самыми дядьками в погонах. Ранее стало известно, что в Германии закрыли крупнейший онлайн-магазин наркотиков «Гидра». Хороший и надежный сервис, получи свой.onion имейл. Vtg3zdwwe4klpx4t.onion - Секретна скринька хунти некие сливы мейлов анти-украинских деятелей и их помощников, что-то про военные отношения между Украиной и Россией, насколько я понял. Известны под названиями Deepweb, Darknet. последние новости Гидра года. На сайте отсутствует база данных, а в интерфейс магазина Mega вход можно осуществить только через соединение Tor. Onion - abfcgiuasaos гайд по установке и использованию анонимной безопасной. Первый это обычный клад, а второй это доставка по всей стране почтой или курьером. Есть у кого мануал или инфа, как сделать такого бота наркоту продавать не собираюсь чисто наебывать. Сообщения, анонимные ящики (коммуникации). Этот и другие сайты могут отображаться в нём. Мета Содержание content-type text/html;charsetUTF-8 generator 22 charset UTF-8 Похожие сайты Эти веб-сайты относятся к одной или нескольким категориям, близким по тематике. Mega darknet market и OMG! Система рейтингов покупателей и продавцов (все рейтинги открыты для пользователей). Точнее его там вообще нет. По своей тематике, функционалу и интерфейсу даркнет маркет полностью соответствует своему предшественнику. В противном случае работа будет осуществляться очень медленно. Foggeddriztrcar2.onion - Bitcoin Fog микс-сервис для очистки биткоинов, наиболее старый и проверенный, хотя кое-где попадаются отзывы, что это скам и очищенные биткоины так и не при приходят их владельцам. На создание проекта, как утверждал Darkside в интервью журналу. Форум Форумы lwplxqzvmgu43uff. Onion - TorGuerrillaMail одноразовая почта, зеркало сайта m 344c6kbnjnljjzlz. Клёво12 Плохо Рейтинг.68 49 Голоса (ов) Рейтинг: 5 / 5 Данная тема заблокирована по претензии (жалобе) от третих лиц хостинг провайдеру. Возможность оплаты через биткоин или терминал.
